Ionic Radii
pyrolite incldues a few sets of reference tables for ionic radii in aangstroms
(Å) from [Shannon1976] and [WhittakerMuntus1970], each with tables indexed
by element, ionic charge and coordination. The easiset way to access these is via
the get_ionic_radii() function. The function can be used
to get radii for individual elements, using a source keyword argument to swap
between the datasets:
import matplotlib.pyplot as plt
import pandas as pd
from pyrolite.geochem.ind import REE, get_ionic_radii
REE_radii = pd.Series(
get_ionic_radii(REE(), coordination=8, charge=3, source="Whittaker"), index=REE()
)
REE_radii
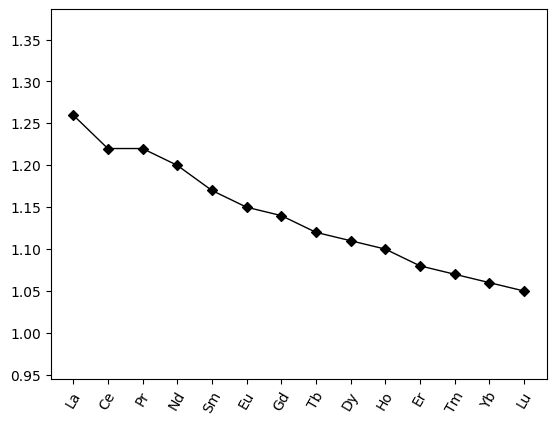
La 1.26
Ce 1.22
Pr 1.22
Nd 1.20
Sm 1.17
Eu 1.15
Gd 1.14
Tb 1.12
Dy 1.11
Ho 1.10
Er 1.08
Tm 1.07
Yb 1.06
Lu 1.05
dtype: float64
REE_radii.pyroplot.spider(color="k", logy=False)
plt.show()
References
- Shannon1976
Shannon RD (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32:751–767. doi: 10.1107/S0567739476001551.
- WhittakerMuntus1970
Whittaker, E.J.W., Muntus, R., 1970. Ionic radii for use in geochemistry. Geochimica et Cosmochimica Acta 34, 945–956. doi: 10.1016/0016-7037(70)90077-3.
- Pauling1960
Pauling, L., 1960. The Nature of the Chemical Bond. Cornell University Press, Ithaca, NY.
See also
Total running time of the script: (0 minutes 0.233 seconds)